A Different Method for AMC

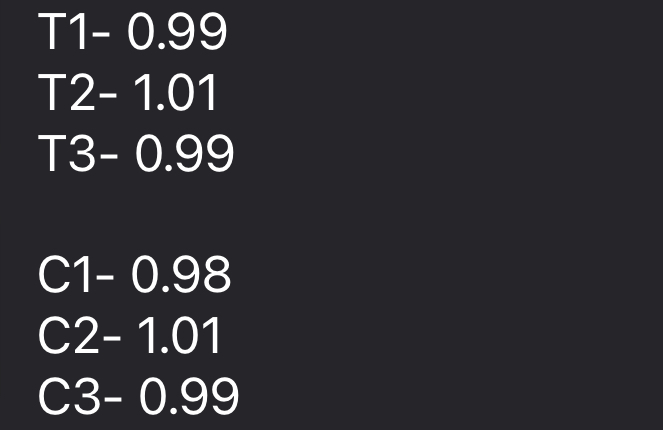
Throughout this semester, we have been focusing on oxidative stress against the bacteria Deinococcus radiodurans. Due to some very consistent results showing that there was no effect on the bacteria when being exposed to hydrogen peroxide, we decided that we would be focusing on bleach. Bleach has a similar effect on cells so we will be moving forward with that. First, we started by doing a dilution series of bleach. We started with a stock of household bleach that was at 5.25%. We wanted a large range to start with just to see if there was any growth, the range went from 0.10%, 0.25%, and 0.50% of 5.25%. The bleach was diluted using nuclease free water. The goal amount of cells was an OD600 value of 1. We added 50uL of cells in TGY at an OD of 1 to 450uL of the respective amount of NaOCl (bleach). After combining, the solutions were then incubated at 30 degree Celsius for 30 minutes. After the exposure, we plated in triplicates for our concentra...